Is Alkaline Water Good?
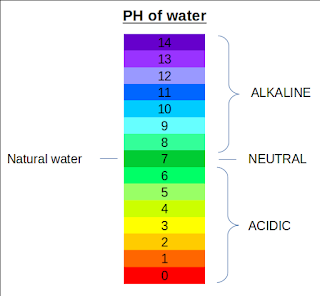
These days we have heard many claims about the alkaline water and its health benefits. Many companies market their product saying it can help in maintaining your body pH level, have anti-ageing properties and prevent chronic disease like cancer. But is alkaline water is good for your health? Let's find out. What is Alkaline Water? Alkaline water is referred to as water having a pH value greater than 7. Alkaline water typically has a pH between 8-9 . pH value is a measure of acidity or basicity of the substance. Pure water free from any kind of impurities has a pH value of 7. Many promoters believe that alkalinity in water can help in neutralizing the excess amount of acid in the body and increase the metabolism rate. What is Body pH and How it is Regulated? The normal range for pH in your body is between 7.35-7.45 so, very slightly alkaline . At times, this balance can be disrupted by our diet and lifestyle. pH can go up or down and this is caused by many factor
